Breaking Down Hydrogen Production Methods

Hydrogen fuel represents an essential component of the transition to renewable energy.
Hydrogen has a virtually unlimited supply, offering the potential for near-endless generation and consumption. Harnessing hydrogen fuel will allow us to reach the net-zero emissions targets set to address climate change over the coming decades.
Hydrogen is an incredibly clean fuel. When hydrogen is consumed in fuel cells, it produces water, which, unlike other fuels, generates pollutants, particles, and other toxins. It can be generated from many sources, including natural gas, nuclear power, biomass, solar, and wind.
The abundant — and often renewable — resources that can be used to create hydrogen make it an attractive fuel option for transportation and electricity generation. It is used in cars and houses, for portable power, and in many other applications. Hydrogen is also leveraged to store, move, and deliver energy produced from different sources.
Hydrogen fuel can be generated in many ways. The most common are thermochemical processes and electrolysis. Others include solar-driven and biological methods.
Let’s break down hydrogen production methods to better understand the path to net-zero emissions.
Thermochemical Hydrogen Production
Thermochemical (sometimes referred to as thermal) hydrogen production processes typically depend on steam. Steam reacts with a hydrocarbon fuel to produce hydrogen. Hydrocarbon fuels that can be leveraged to produce hydrogen include natural gas, diesel, renewable liquid fuels, gasified coal, or gasified biomass.
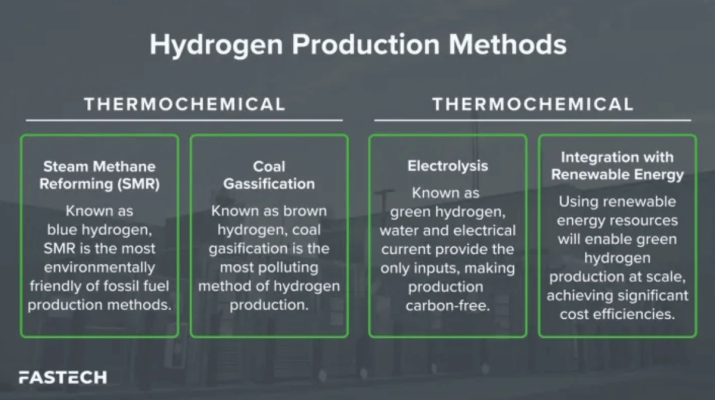
Traditionally, hydrogen production relied on thermochemical processes, commonly using fossil fuels. Let’s review the two primary methods of hydrogen production using thermochemical processes.
Steam Methane Reforming (SMR)
Steam methane reforming, (also referred to as natural gas reforming and blue hydrogen), accounts for 95 percent of today’s hydrogen production in the United States.
SMR steam-heats methane sourced from natural gas to temperatures between 700o-1000o C.
When the two combine and are pressurized, the chemical reaction results in:
- Hydrogen (H)
- Carbon monoxide (CO)
- Carbon dioxide (CO2)
Next, steam is combined with carbon monoxide to produce more hydrogen and carbon dioxide via water-gas shift reaction. The final process, pressure-swing absorption, removes all elements aside from the hydrogen.
Of the hydrogen production methods involving fossil fuels, SMR is the most environmentally friendly. However, it still requires significant technologies and localized infrastructure to minimize carbon emissions. These hydrogen production technologies, known as carbon capture, reduce the emissions that escape into the atmosphere.
Furthermore, the potential release of methane throughout the process has much more damaging climate change effects than CO2. SMR also requires substantial energy (usually created by consuming fossil fuels) to heat water and make the necessary high-temperature steam.
Coal Gasification
The coal gasification method also creates hydrogen, CO, and CO2, but is achieved by heating coal until it creates a syngas (a fuel-gas mixture). As the most polluting method of hydrogen production — releasing 10 to 12 tons of CO2 for every ton of hydrogen — the product of coal gasification is known as brown hydrogen.
Read more: The Colors of Hydrogen, Explained
Electrolytics
A process known as electrolysis makes it possible for water to be separated into oxygen and hydrogen. This occurs in an electrolyzer, which functions like a fuel cell acting in reverse. In this case, instead of using the energy of a hydrogen molecule like a fuel cell does, the electrolyzer generates hydrogen from the molecules in water.
The electrolytic process represents the most sustainable hydrogen production method currently available to perform at scale. When this process is combined with renewable sources such as wind and solar energy, it is known as “green hydrogen” for its carbon-free operation.
As a result, electrolytics represent one of the most significant sustainability transformations we can make in our energy sector.
Electrolysis explained
Electrolysis splits water molecules into hydrogen and oxygen via electrical current. This process occurs within equipment known as an electrolyzer. Because water and electrical current provide the only inputs, no carbon or other GHG emissions are released — only water vapor.
Fuel cells perform similar chemical processes to convert hydrogen gas into electricity without needing batteries.
Integration With Renewable Energy
For electrolysis to produce green hydrogen, renewable energy sources must provide the electricity input. Therefore, electrolysis will grow in popularity as we continually convert more electricity production to wind, solar, and other sustainable methods.
Electrolysis will enable green hydrogen production at a scale that achieves significant cost efficiencies, as demonstrated by SoCalGas’s recently proposed Angeles Link project. This system is expected to replace 25% of the natural gas SoGalGas delivers to the LA Basin for electricity generation.
Solar-Driven Processes
Solar-driven hydrogen generation processes leverage light to produce it. There are three primary types:
- Photobiological processes use the natural photosynthetic activity of bacteria and green algae to generate hydrogen.
- Photoelectrochemical processes use specialized semiconductors to separate water into hydrogen and oxygen.
- Solar thermochemical hydrogen production leverages highly concentrated solar power to generate water-splitting reactions. Metal oxides are often incorporated into this type of hydrogen production.
Biological Processes
Biological hydrogen generation processes use microbes, including bacteria and microalgae, to produce it through biological reactions. In microbial biomass conversion, the microbes break down organic matter, often biomass or wastewater, to create hydrogen. Microbes use sunlight as the energy source in photobiological processes.
Comparison of the Different Hydrogen Generation Methods
Deciding between electrochemical and thermal hydrogen production must take into account many factors, including:
- Resource availability
- Energy efficiency
- Environmental impact
Currently, many experts view the complementarity of these technologies as more beneficial rather than seeing them as direct competitors.
Electrochemical hydrogen production is typically used in applications where high-purity hydrogen is preferred and electricity generated from renewable sources is available. This is required to produce green hydrogen, which is critical for reducing harmful carbon emissions in industries like transportation. In today’s global environment, where people and governments are concerned about global warming, electrochemical hydrogen production is viewed as a growing, vital part of fuel production.
Meanwhile, thermochemical hydrogen production is necessary for large-scale applications and is required for industries where a massive amount of hydrogen is required, even if it can harm the environment, such as in the chemical industry and petroleum refining.
Over the long term, the goal is to replace thermochemical hydrogen production with electrochemical processes.
FASTECH: Experts in Hydrogen Infrastructure EPC+M
Electrolysis’s sustainable hydrogen production methods provide a clear path towards a sustainable future and offer a direct replacement for thermochemical technologies.
Until production technologies can operate efficiently at much smaller scales, hydrogen gas will be created at dedicated plants with necessary transport infrastructure.
When it comes to hydrogen infrastructure engineering, procurement, construction, and maintenance (EPC+M), FASTECH is the partner you’ve been looking for. We’re the leading provider of end-to-end alternative energy solutions that reduce your carbon footprint and create a more sustainable tomorrow.
Contact us to find out how we can help you embrace sustainable hydrogen production methods.